Abstract
BACKGROUND:Pyruvate kinase (PK) deficiency is a congenital hemolytic anemia caused by mutations in the PKLR gene, leading to a deficiency of the glycolytic enzyme red cell PK (PK-R). Current treatments for PK deficiency are supportive only. AG-348 is an orally available small-molecule allosteric activator of PK-R that activates the wild-type and a range of mutated PK-R enzymes in vitro, and increases PK-R activity in red blood cells from patients with PK deficiency ex vivo (Kung C et al. 2017 Blood in press).
AIM:To report updated data from the ongoing DRIVE PK study (ClinicalTrials.gov NCT02476916), an open-label, dose-ranging trial of AG-348 in nontransfusion-dependent adults with PK deficiency.
METHODS:Patients were randomized to 50 mg or 300 mg AG-348 orally twice daily (BID) for a 6-month Core Period; Core Period completers may enter a 2-year Extension Period. Dose modifications were allowed. For this study, nontransfusion-dependence is defined as ≤3 units of red blood cells transfused in the 12 months preceding the first dose and no transfusions in the 4 months preceding the first dose. Patients are followed up at Weeks 1, 2, 3, 6, 9, 12, 16, 20, and 24 in the Core Period, and subsequently every 3 months in the Extension Period. Safety and tolerability (primary endpoint), pharmacokinetics, pharmacodynamics, and hematologic parameters including hemoglobin (Hb) are assessed. Here, we report safety and efficacy data based on the data cutoff date of March 27, 2017; updated data from the Core and Extension Periods and complete data from the Core Period will be available at the meeting. The rate of glycolytic metabolism in peripheral blood samples was measured in a subset of patients before and after treatment to assess PK-R pathway activation.
RESULTS:Enrollment was completed in November 2016 (n=52; median baseline age 34 years; 62% men; 81% white). As of March 27, 2017, 29 patients had completed the Core Period, 15 were ongoing in the Core Period, and 8 had discontinued the Core Period owing to adverse events (AEs) (n=3), investigator decision (n=2), or consent withdrawal (n=3). Of the 29 patients completing the Core Period, 24 entered the Extension Period: 21 were ongoing and 3 had discontinued the Extension Period owing to investigator decision (n=2) or consent withdrawal (n=1). Median (range) treatment duration was 24.9 (12.9-76.9) weeks. As a result of protocol-specified dose modifications, subjects received doses in the range between 5 mg once daily and 300 mg BID.
AG-348 had an acceptable safety profile. The most common AEs (occurring in >10 patients) were headache (44%, n=23), insomnia (39%, n=20), and nausea (37%, n=19). Testosterone levels increased because of known aromatase inhibition; however, most values remained within the normal range. Six drug-related serious AEs occurred in 5 patients (withdrawal hemolysis followed by anemia; anemia; osteoporosis; hypertriglyceridemia; pharyngitis).
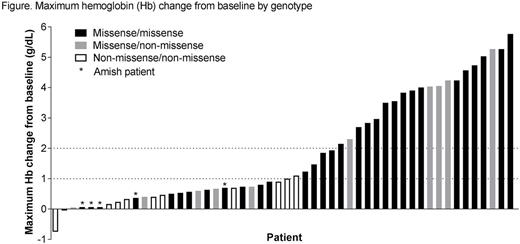
Of 52 patients, 25 (48%) experienced a maximum Hb increase >1.0 g/dL (mean maximum increase 3.5 g/dL, range 1.1-5.8 g/dL). In most cases, Hb increases were rapid and sustained, and seen across a wide dose range from 5 to 300 mg BID. Some genotype-Hb response correlations were observed (Figure), in which those with at least 1 missense mutation were more likely to have an Hb increase. Hemolysis markers (reticulocytes, indirect bilirubin, haptoglobin) improved in patients who experienced a maximum Hb increase >1.0 g/dL. In a subset analysis (n=8), a positive correlation was observed between increased Hb and activation of the PK-R pathway, as measured ex vivo via incorporation of labelled glucose into lactate, the end product of the PK reaction.
CONCLUSIONS:AG-348 is a novel, first-in-class PK-R activator in clinical testing for PK deficiency. The ongoing DRIVE PK study has completed enrollment, and final Core Period data will be available at the time of presentation. Chronic daily dosing with AG-348 has been generally well tolerated and has resulted in clinically relevant, durable increases in Hb and reductions in markers of hemolysis across a range of doses. These data support the potential of AG-348 as the first disease-altering therapy for PK deficiency.
Grace: Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Layton: Alexion: Other: UK PNH Physician Network; Agios: Consultancy, Speakers Bureau; UCB: Consultancy. Galactéros: Addmedica: Membership on an entity's Board of Directors or advisory committees. Barcellini: Alexion: Honoraria; Agios: Honoraria, Research Funding; Novartis: Honoraria. Van Beers: Agios: Consultancy. Yaish: Baxalta, Bayer and Octapharma, CSL behring: Consultancy, Membership on an entity's Board of Directors or advisory committees; Baxalta: Honoraria, Membership on an entity's Board of Directors or advisory committees. Ravindranath: Agios: Other: Site investigator. Kuo: Celgene: Consultancy, Other: Site-PI on ACE-536-B-THAL-001; Novartis: Consultancy, Honoraria; Pfizer: Other: Site-Pi for GMI1070 study; Phoenicia Biosciences: Other: Scientific collaboration; Abfero: Other: Scientific collaboration; Alexion: Consultancy, Honoraria, Other: Site-PI for ALXN1210-PNH-301, ALXN1210-PNH-302, ALXN1210-aHUS-311, PKD natural history study, aHUS natural history study (M11); Agios: Consultancy, Other: Site-PI for AG-348-003 and PKD natural history study. Sheth: Novartis, Celgene, ApoPharma, Bluebird Bio: Consultancy. Kwiatkowski: Ionis: Consultancy, Honoraria; Agios: Consultancy, Honoraria; Novartis: Research Funding; Apopharma: Research Funding; Bluebird Bio: Research Funding. Silver: Agios: Consultancy. Kung: Agios: Employment, Equity Ownership. Cohen: Agios: Consultancy. Yang: Agios: Employment, Equity Ownership. Kosinski: Agios: Employment, Equity Ownership; General Electric: Equity Ownership. Hua: Agios: Employment, Equity Ownership. Barbier: Agios: Employment, Equity Ownership. Glader: Agios Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal